Welcome to our CSET Science Test prep page. We’ll be introducing you to the domains and key concepts you need to know to pass this exam. This is one of the free resources we provide so you can see how prepared you are to take the official CSET Science Test.
The California Subject Examinations for Teachers (CSET) were developed as a way to test the content knowledge of prospective teachers. The CSET Science exam is a test taken by California educators wanting to teach science at the secondary level. This exam tests knowledge of the following: life science, physical science, earth science (including space), engineering design and applications, scientific practices and other crosscutting concepts.
Quick Links to Help You Navigate This Page
- CSET Science Test Information
- Subtest I: General Science
- Subtest II: Life Sciences
- Subtest II: Chemistry
- Subtest II: Earth and Space Sciences
- Subtest II: Physics
- General Science Practice Questions and Answers
- Life Sciences Practice Questions and Answers
- Chemistry Practice Questions and Answers
- Earth and Space Sciences Practice Questions and Answers
- Physics Practice Questions and Answers
CSET Science Test Information
Format:
The CSET Science is computer based only and is made up of 2 separate subsets: Subtest 1 (215), Subtest 2 (217-220). The first subset covers general science and the second, a specific concentration (Life Sciences (217), Chemistry (218), Earth and Space Sciences (219), and Physics (220).
Subtest I: 100 multiple-choice questions and 4 constructed-response questions. Four hours of test taking time are given.
Subtest II: 50 multiple-choice questions and 3 constructed-response questions. Two hours of test taking time are given.
If you take both subtests in a single session, you are given 6 hours.
Cost:
If both Subtests are taken together, the fee is $267.
If only Subtest I is taken- $133
If only Subtest II is taken- $134
Scoring:
To pass, a test taker must score at least 220 in each of the subsets.
Pass rate:
According to the CTC Annual Passing Rate Report pass rates for specific CSET science subsets range between 63-81%
Study time:
There is no magic number of hours that one has to spend studying to pass a CSET exam. Instead of focusing on a specific amount of study time, many future teachers find it more beneficial to make sure the time they do spend going over test prep materials is focused and not ‘crammed.’ Gather your study materials, make a study schedule, and stick to it until your exam day arrives.
What test takers wish they would’ve known:
The biggest regret of test takers who didn’t meet the minimum 220 score is not scheduling enough study time. Even if you were a strong science student in school, you will need practice with specific exam content before taking the CSET Science exam.
Information and screenshots obtained from the California Educator Credentialing Assessments website:
CSET Science Key Concepts to Know
Subtest I: General Science
Subtest I: General Science has 100 multiple-choice questions and 4 constructed-response questions. You will have 4 hours to complete this subtest.
This subtest can be neatly divided into 4 domains:
- Scientific Practices, Engineering Design and Applications, and Crosscutting Concepts
- Physical Sciences
- Life Sciences
- Earth and Space Sciences
Scientific Practices, Engineering Design and Applications, and Crosscutting Concepts
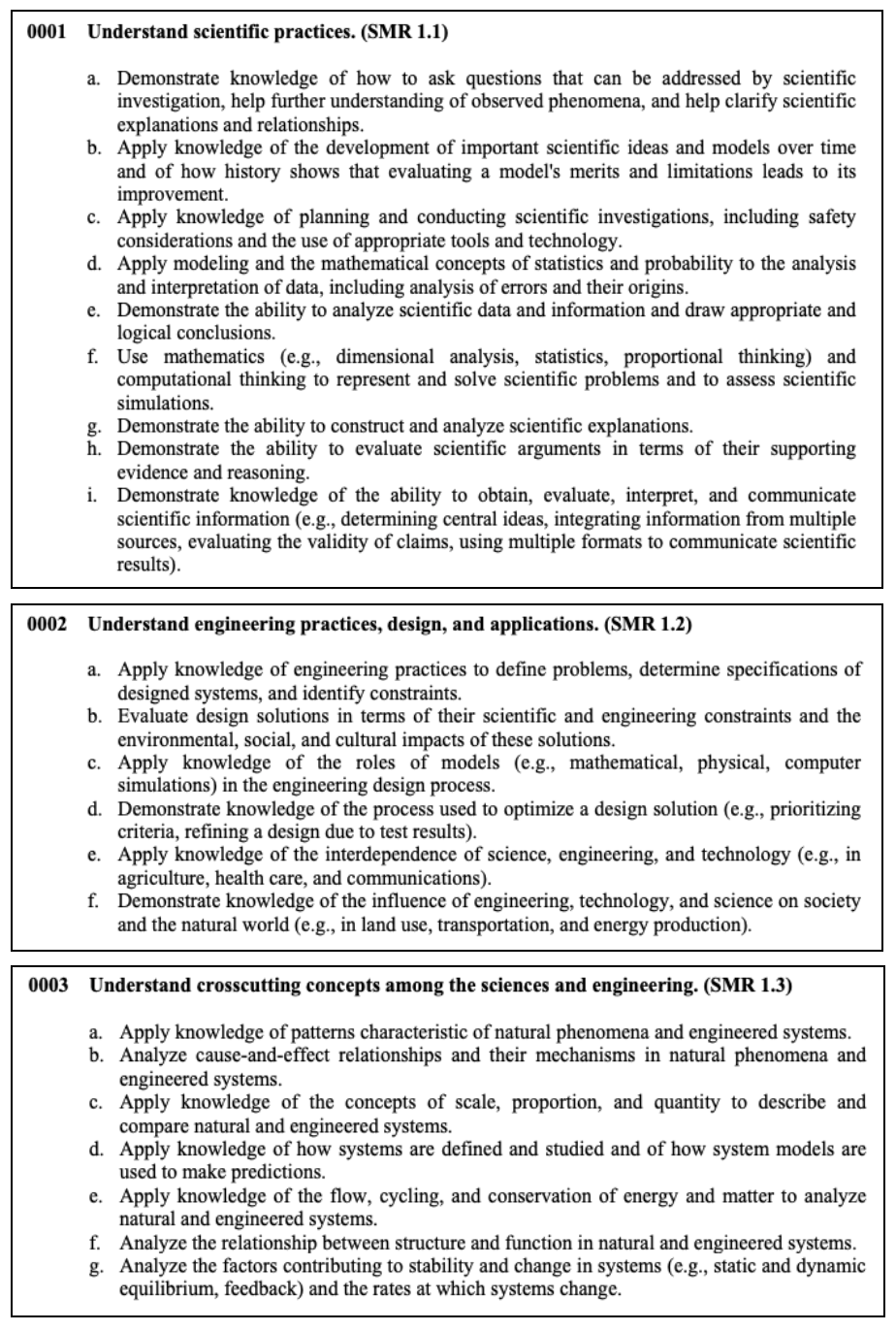
This section tests your knowledge of scientific investigations, engineering practices, and applying crosscutting concepts in a real-world setting.
Let’s discuss some concepts that will more than likely appear on the test.
The Scientific Method
The scientific method is probably the most widely taught topic in secondary science labs. Science and the way we teach is ever changing, but this process for investigating natural phenomenons remains a staple in high school science classrooms. Students use the scientific method to understand and answer questions about the natural world.
Here’s a general idea of what these steps might look like in a secondary environment.
- Make an Observation/Ask a Question.Let’s say a student, Laura, is chewing gum in class. Her best friend, London, is chewing a piece as well. London’s bubbles seem to be a lot bigger than Laura’s. Laura wonders if her friend’s brand of gum makes larger bubbles.
- Gather Information.During study hall, she asks London what kind of gum she’s chewing. She says it is called Bubble Bubble and offers Laura a few pieces. Laura looks on her own wrapper and sees that she was chewing Apple Bubble Gum.
- Make a Hypothesis (educated guess).Laura forms a hypothesis–that brand B (Bubble Bubble) makes bigger bubbles than brand A (Apple Bubble.)
- Experiment/Test.When they get free time in the lab, Laura decides to test her hypothesis. She takes turns blowing bubbles with each of the gum brands and has London measure. She repeats this several times. Then, she has London to do the same. They also have their friend Charlie conduct the experiment.
- Analyze Your Test Results.After measuring, Laura looks at her measurements. She finds a few things. First, London blew the biggest bubbles of all, but not with Bubble Bubble (brand B). All three of the students blew bubbles over two inches with Apple Bubble (brand A). London’s biggest ‘Apple bubble’ was 2.9 inches. No one blew a bubble over two inches with Bubble Bubble.
- Present a Conclusion.By using the scientific method, Laura was able to put her hypothesis to rest. London’s bubble gum didn’t make bigger bubbles, it seemed that London was just better at blowing bubbles.
Maintaining Balance: Static and Dynamic Equilibrium Stability is used to describe a condition in which some aspects of a system are unchanging (all forces acting on an object are balanced). If a system is stable, any small disturbances to the system will fade away and the system will return to a state of equilibrium (balance).
One type of stability is called static equilibrium , also known as mechanical equilibrium . This is the simplest to understand as it occurs in objects that are at rest. Examples include a ladder lying against a house, the CO2 quantity in a bottle of unopened soda. No “dynamics” are involved here.
Dynamic equilibrium represents a state of stability between continuing processes. One good example is a dam that has water constantly flowing in and out.
Dynamic equilibrium is also considered by engineers when building various structures. These engineers must calculate forces that will be applied to these frameworks and make sure that they are strong enough to maintain balance against whatever force being exerted.
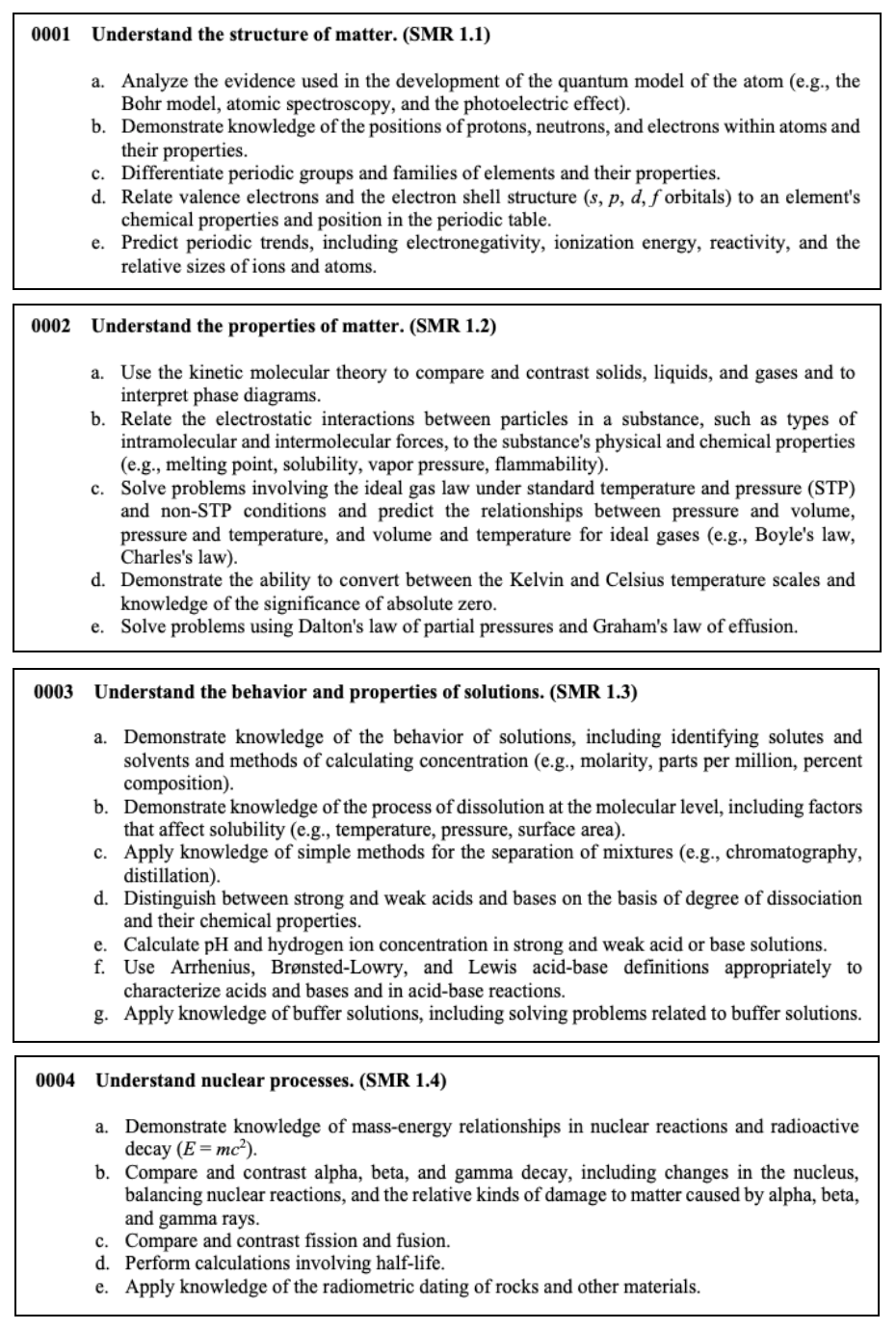
Physical Sciences
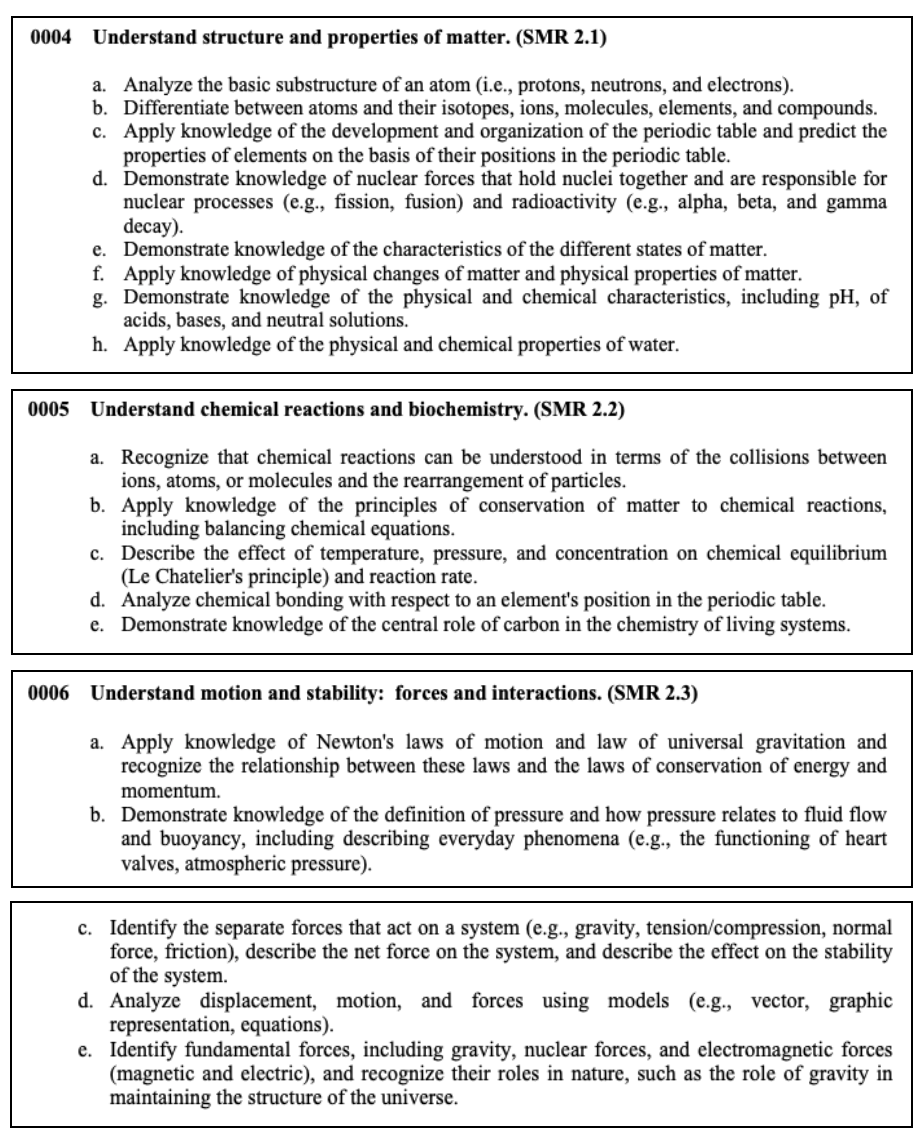
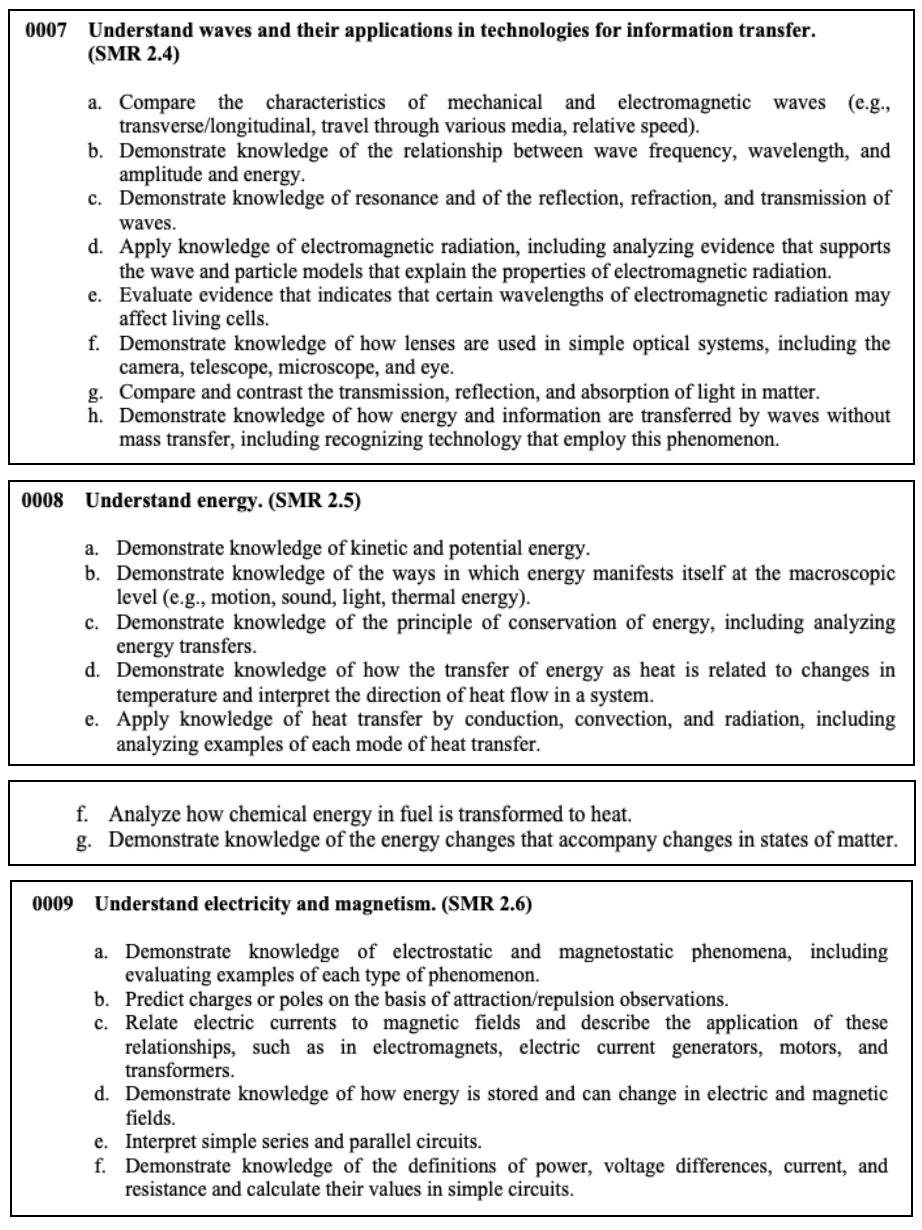
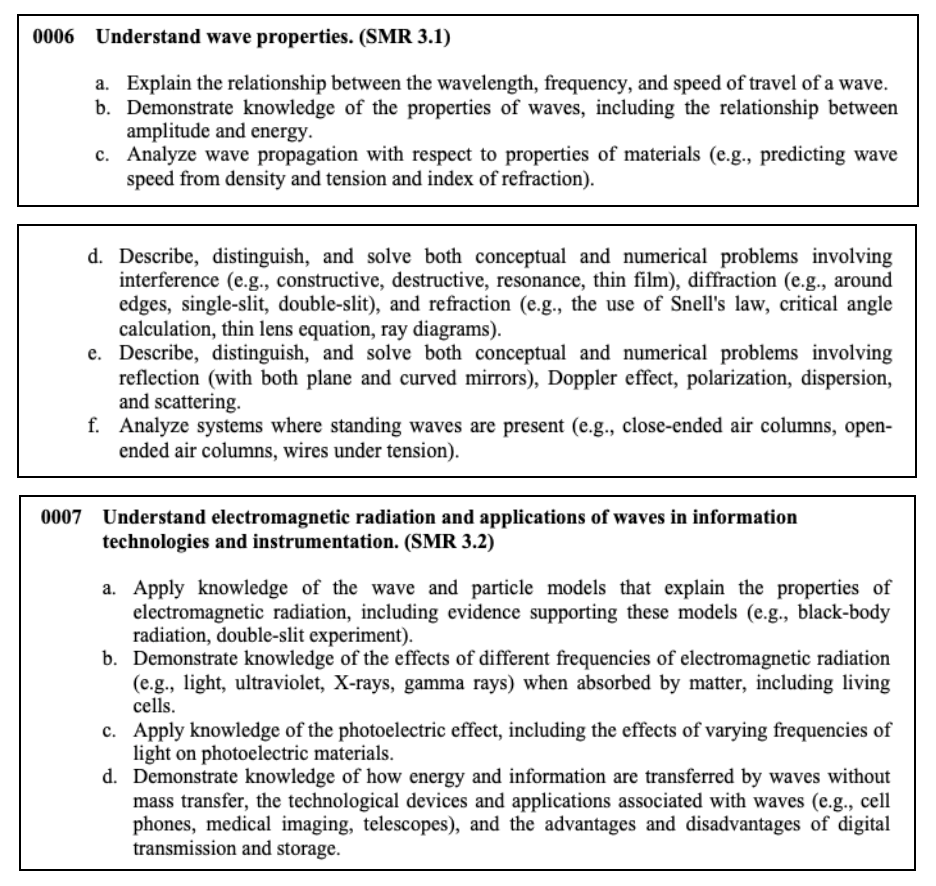
This section tests your knowledge on the properties of matter, chemical reactions, interacting forces, wave transfers, as well as electricity and magnetism.
Here are some concepts that you may see on the test.
Nuclear Processes
Atoms make up everything in the universe. In the center of each atom is a
nucleus, the center part where all of an atom’s mass and energy are contained.
This energy is known as nuclear energy .
Nuclear processes , also known as nuclear reactions , occur when nuclear particles, or two nuclei, interact. The most common examples of a nuclear reaction are fission and fusion.
Fission: Dividing Atoms
By definition, fission means ‘splitting apart.’ With nuclear fission, atoms split apart and the effect (heat) is released. When this process happens in a manner that is slow and controlled, the large amounts of energy produced can be used to generate electricity.
A common example of how fission is used involves power plants. These nuclear
power plants use control rods of the element uranium to
heat. This creates steam from water which, in turn, powers electrical generators. Nuclear fusion can also be used to power submarines.
Fusion: Joining Atoms
A second nuclear process, fusion , occurs when two atoms join together, forming a larger atom. The ‘fusing’ of these two nuclei also results in the release of huge amounts of energy. Unlike fission, scientists have not figured out how to control fusion. However, we do know of several things that result from the joining of atoms.
One of the best-known examples involves our greatest energy source of all: the Sun. Fusion actually occurs deep inside all-stars as their atoms are constantly being changed into helium gas. Through fusion, light and energy are produced in the form of a star.
Types of Energy Energy is the ability to do work. It cannot be created or destroyed but always exists in some form or another. Energy can be transferred from one form to another.
There are two basic forms of energy: potential and kinetic . Potential energy is stored power. This type of energy is energy at rest. It might seem ‘hidden’, but it still exists, ready to act. Some examples of potential energy include:
- Elastic Potential Energy– energy stored inside a spring or a rubber band
- Gravitational Potential Energy– energy built up in a car sitting on top of a tall hill
- Chemical Potential Energy– stored energy contained inside coal or wood
Kinetic energy is energy that an object has while in motion. Some of the most common types of kinetic energy and examples are:
- Sound Energy– energy connected to a person’s voice, the sound of a buzzing bee, or the snapping of one’s fingers
- Thermal Energy– energy contained in a hot cup of coffee, hot springs, something being baked, or a heated swimming pool
- Radiant (Electromagnetic) Energy– energy of sunshine, X-rays, radio waves/signals, headlights on a vehicle
- Electrical Energy– energy harnessed through lightning, contained in doorbells, and even inside your brain
- Mechanical (Moving) Energy– satellites orbiting the earth, someone jumping rope, or a car driving along a highway
Life Sciences
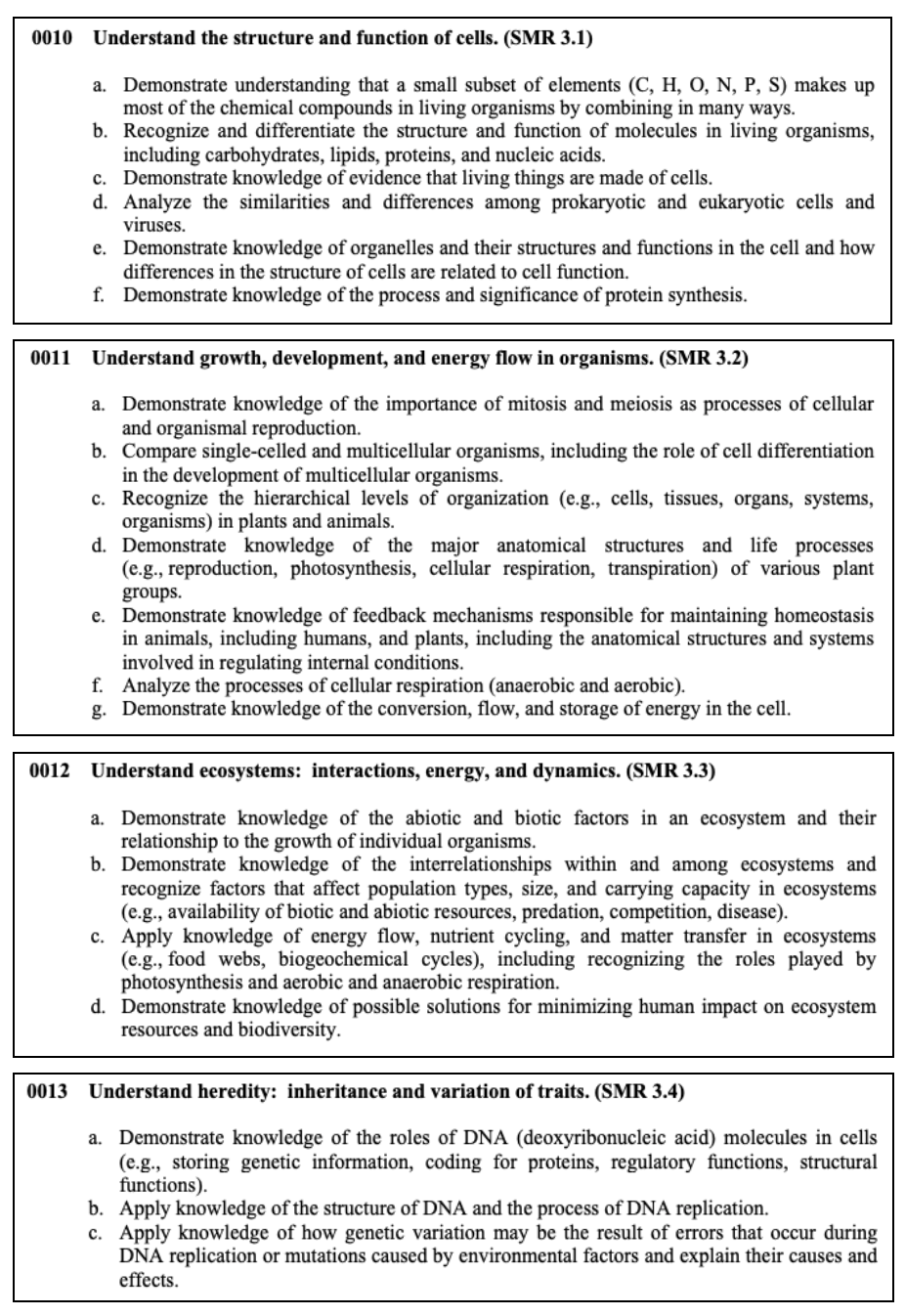
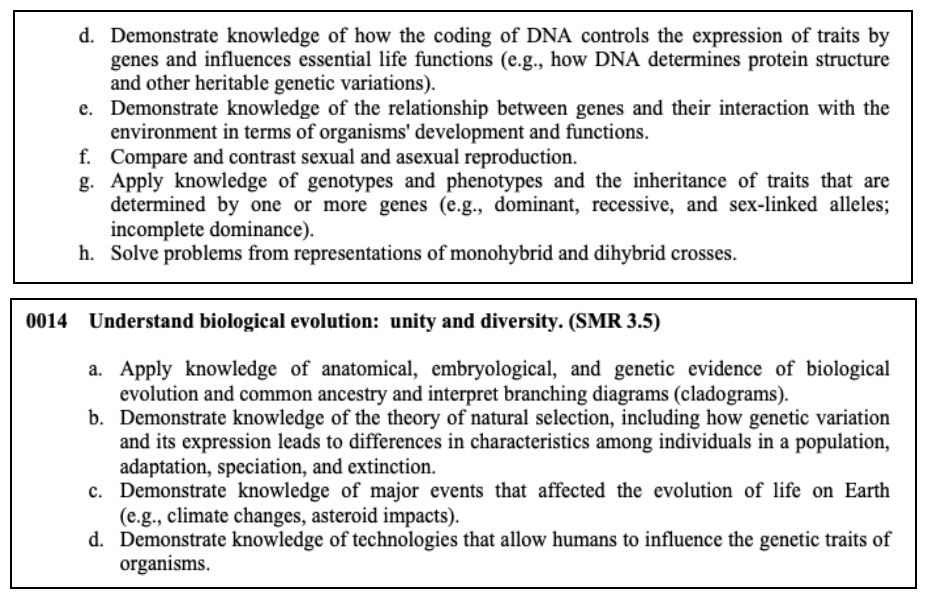
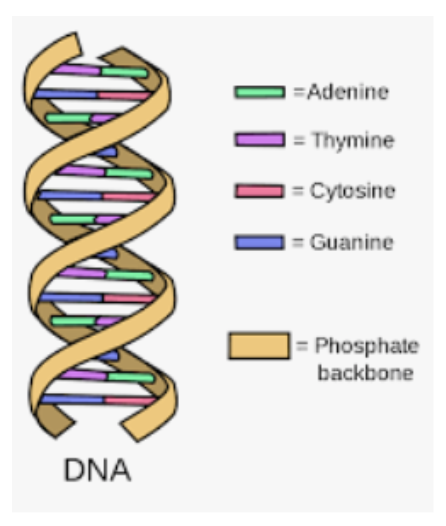
This section tests your knowledge on cells and how they function, the development of organisms, heredity, and DNA, interactions in ecosystems, and how things have evolved over time.
Let’s look at some concepts that are guaranteed to come up on the test.
Processes of Cellular Respiration
The term cellular respiration is used to describe the way living things
break down food molecules (glucose) into chemical energy. All forms of life except viruses go through this process. The overall equation for cellular respiration is:
C₆H₁₂O₆ + 6O₂ ⇒ 6CO₂ + 6H₂O + energy
This breakdown takes place in two main ways:
- The first process is also called aerobic respiration, and it requires oxygen (think air = aer). Glycolysis, Krebs cycle, and electron transport chain are the three steps of this type of respiration. Your brain, liver, and other organs undergo this process.
- Anaerobic respirationneeds no oxygen for the break down to take place. Both anaerobic and aerobic respiration begin with the anaerobic breakdown of glucose by glycolysis. But instead of moving into the Krebs cycle, fermentation comes next. Unicellular organisms, like those that produce yeast, use anaerobic respiration.
Food Webs
All living things need energy to survive. To get this energy, they either make or consume food. This is an interdependent system. Each living thing is a part of a food chain like the one below.
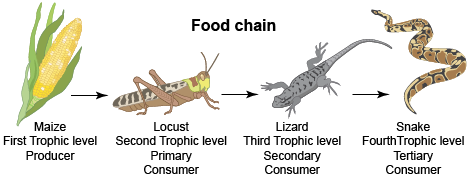
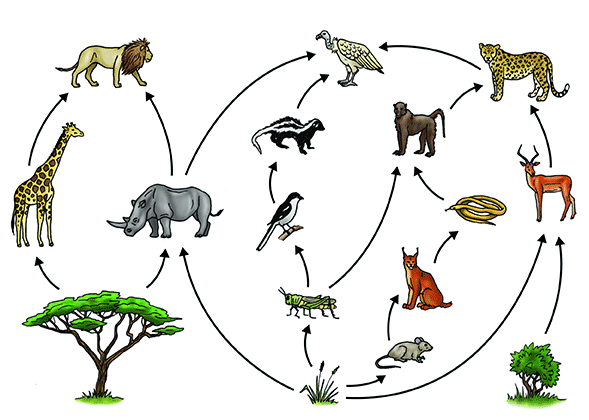
Earth and Space Sciences



This section tests your knowledge on Earth and our universe as a whole, tectonic plates and their interactions, natural hazards, resources, and weather/climate.
Here are some specific concepts that will more than likely appear on the test.
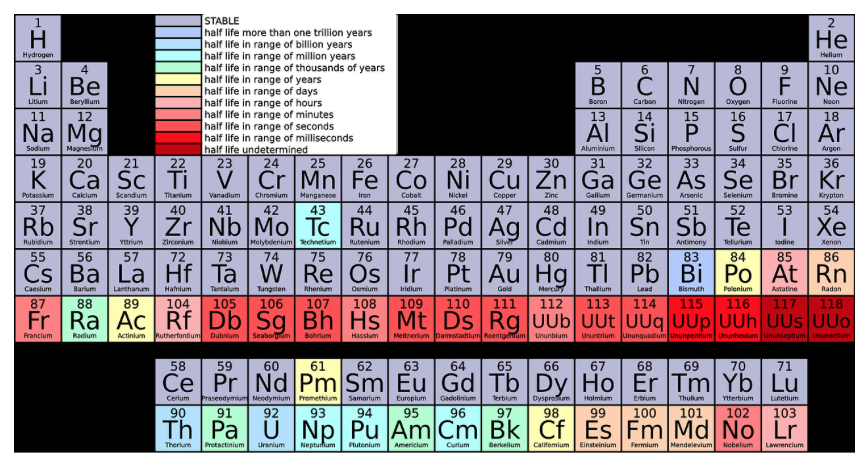
Stars
By definition, stars are huge spheres of gas. Created by nuclear fusion, stars are held together by their own gravity. Our galaxy, the Milky Way, contains billions of stars. The Sun, a G2 yellow dwarf star, is the closest star to Earth.
Astronomers classify stars based on their properties. Three important properties include color, size, and luminosity. Let’s review each property.
Color
A star gets its color based on its surface temperature. Red dwarf stars are the coolest in our universe. Blue stars burn the hottest. Our star, the Sun, is a G star that is classified as ‘white’, even though it gives off a yellowish glow. It falls in the middle in regard to surface temperature.
Size
Just as stars come in a variety of colors, they also come in various sizes. Small neutron stars are sometimes as small as 30 km (around 18 miles) in diameter.
Stars tend to grow in size as they grow in mass. Large gas giants can be over 1,500 times larger. The Sun is 695,000 kilometers (431,852 miles) in diameter. That sounds huge, right? When compared to the red giant, Betelgeuse, our own star is a 1,000 times smaller.
Luminosity
Also known as a star’s intrinsic brightness, a star’s luminosity is one of the most commonly confused classifying traits. Most people confuse luminosity with apparent magnitude, which is how bright it appears from Earth. But scientists use the term luminosity to describe intrinsic brightness–how bright it is on the inside.
A star’s luminosity is measured by:
- surface temperature
- radius
Keeping this in mind, do you know the answer to this question? If a scientist is comparing two stars and both have the same surface temperature, which has greater luminosity? The answer– whichever has the greatest surface temperature.
It’s also important to remember that a star’s light spectrum and brightness can be used to identify compositional elements, movements, and distance from Earth. For example, the stars that are the brightest (greatest luminosity) are the closest to Earth in distance.
The Water Cycle
The most renewable of all resources, water is continually regenerated through the stages of the water cycle . Water always exists in these three places: the ocean, the land, and the atmosphere. It also takes on many forms. These include lakes, rivers, and even sheets of ice.
This is how it works:
- The Sun heats up water inside the ocean, lakes, rivers, etc. Through this process called evaporation ,steam is formed. This steam is also called water vapor, and it rises into the air. Plant transpiration helps this process.
- Next, the gaseous form of water (vapor) changes back into a liquid in the sky. This process, condensation , is how clouds are formed.
- As more and more water is formed inside of the clouds, precipitation begins. The water drifts back down, filling the lakes and oceans once again.
This cycle continues to repeat. We must note that there is a relationship between the most common water reservoirs (things on the surface of Earth) and those below. Like streams, flooded plains, and swamps, subsurface reservoirs also play a hand in the water cycle. These include areas that were too saturated with liquid, allowing water to sink deep down within to be stored.
And that’s some basic info about CSET Subtest I: General Science.
CSET Science Practice Questions
Question 1
Which of the following is not an example of a scientific model?
- a graph of monthly precipitation
- a flowchart of the life cycle of a grasshopper
- the formula for Newton’s second law of motion
- a piece of lunar rock
Correct Answer: 4
Explanations:
- A graph is an example of a visual model or representation of data or phenomena.
- A flowchart is a visual model or representation of phenomena.
- The formula for Newton’s second law of motion is a mathematical model or representation of phenomena.
- A piece of lunar rock is not a model; it is an actual sample.
Question 2
Which type of investigation has one manipulated variable, but the researcher chooses experimental groups rather than assigning them randomly?
- Quasi-experimental research
- Correlational research
- Single-variable research
- Experimental research
Correct Answer: 1
Explanations:
- Quasi-experimental research involves the researcher choosing experimental groups. For example, a researcher may choose two different schools and implement a healthy eating campaign in one school and not in the other.
- Correlational research involves the manipulation of two different variables and investigates possible relationships between the variables.
- Single-variable research has only one manipulated variable and no other controlled conditions. A researcher making observations before and after asking a question is an example of this type of research.
- Experimental research takes place under carefully controlled conditions.
Question 3
Which of the following correctly explains the difference between heat and thermal energy in science?
- An object has thermal energy but heat is transferred between objects.
- An object has heat but thermal energy is transferred between objects.
- There is no difference. Heat and thermal energy are the same thing.
- Heat is a measure of how hot an object is, whereas thermal energy is a measure of how fast the object’s molecules move.
Correct Answer: 1
Explanations:
- The energy transferred because of a temperature difference is heat. The internal energy of an object is thermal energy.
- The terms “heat” and “thermal energy” are reversed here.
- Heat and thermal energy are not the same. One is a process, the other is a quantity.
- Both definitions are describing temperature.
Question 4
Which of the following will give off energy if used as fuel in a fission reaction?
- helium (He)
- beryllium (Be)
- thorium (Th)
- hydrogen (H)
Correct Answer: 3
Explanations:
- Helium is a very light element and is not suited for fission.
- Beryllium is a light element and is not suited for fission.
- Fission is the splitting of heavy elements into lighter elements. All elements heavier than iron emit energy during fission.
- The nucleus of hydrogen is a single proton. Hydrogen cannot fission.
Question 5
Which of the following pathways provides organisms with the most energy?
- Glycolysis
- electron transport chain
- Krebs cycle
- fermentation
Correct Answer: 2
Explanations:
- Glycolysis results in the net gain of 2 ATP.
- The electron transport chain results in the production of 34 ATP.
- The Krebs cycle results in the production of 2 ATP for every molecule of pyruvate used.
- Fermentation results in the net gain of 2 ATP.
Question 6
Which of the following is true of the light-dependent and light-independent reactions of photosynthesis?
- The light-dependent reactions take place in the thylakoid membrane, and the light-independent reactions take place in the stroma.
- The light-dependent reactions produce some reactants needed for the light-independent reactions, and the light-independent reactions produce some reactants needed for the light-dependent reactions.
- The light-dependent reactions require energy from the Sun, and light-independent reactions do not.
- All of the above are true.
Correct Answer: 4
Explanations:
- The photosystems for the light-dependent reactions are located in the thylakoid membrane.
- The light-dependent reactions produce NADPH and ATP, and the light-independent reactions produce NADP+ and ADP + P.
- The light-independent reactions, also called the Calvin Cycle, are so named because they do not require solar energy. However, the light-independent reactions do require products made in the light-dependent reactions, so they cannot continue in the dark indefinitely.
- All the statements are true of the light-dependent and light-independent reactions.
Question 7
Which of the following statements most accurately describes an igneous rock?
- a light, very porous rock with visible holes, grains that are too small to see, and no fossils
- a rock with wavy bands of minerals that is very hard and has visible crystals
- a rock that is soft and grainy
- a rock that appears to have been formed from cemented pieces of other rocks
Correct Answer: 1
Explanations:
- A light, porous rock with visible holes, small grains, and no fossils is most likely pumice, an igneous rock. Igneous rocks never contain fossils. They can be porous, glassy, or crystalline depending on where and how they formed.
- Very hard rocks with visible crystals and wavy bands of minerals are metamorphic rocks.
- Soft, grainy rocks, such as shale or sandstone, are sedimentary rocks.
- Rocks formed by cementation of other particles are sedimentary rocks.
Question 8
Which of the following soil types is best for general gardening?
- sand
- peat
- clay
- loam
Correct Answer: 4
Explanations:
- Sand does not hold water and therefore makes it difficult for plants to remain moist and find nutrients.
- Peat is acidic and is used mainly as a soil amendment.
- Clay can be so dense that roots have a hard time penetrating a clay soil.
- Loam is best for gardening because it holds water and nutrients. It is a mix of sand and silt with some clay.
Question 9
In plate tectonics theory, the term “plastic” refers to:
- a solid material that can flow.
- a polymer.
- a dense, viscous liquid.
- molten rock.
Correct Answer: 1
Explanations:
- The asthenosphere, or mantle beneath the tectonic plates, is solid but is hot enough to slowly flow and push on the tectonic plates. The asthenosphere has a plastic quality.
- Some polymers are used for man-made plastics, but this is not the meaning of the term as it is used in plate tectonics theory.
- In plate tectonics theory a plastic is a solid, not a liquid.
- Molten rock is liquid, whereas a plastic material is solid.
Question 10
How do the nervous system and endocrine system work together to maintain homeostasis?
- The endocrine system receives and interprets information, and the nervous system provides the necessary chemicals for the body’s response to the information.
- The nervous system receives and interprets information, and the endocrine system provides the necessary chemicals for the body’s response to the information.
- The nervous system provides stabilizing responses in a negative feedback loop, and the endocrine system provides accelerating responses in a positive feedback loop.
- The nervous system and the endocrine system do not work together to maintain homeostasis.
Correct Answer: 2
Explanations:
- The nervous system receives and interprets information, and the endocrine system provides the chemicals for the body’s response
- The nervous system governs the body’s response, and the endocrine system helps achieve the response.
- The nervous and endocrine systems can each be involved in both negative and positive feedback loops.
- The nervous system and endocrine system work closely together to maintain homeostasis.
Subtest II: Life Science Practice Test
Question 1
Which of the following choices best completes the analogy listed below?
Lipids are to storing energy as nucleic acids are to ____.
- building and repairing cell parts
- providing quick energy
- speeding up chemical reactions
- carrying genetic material
Correct Answer: 4
Explanations:
- Proteins are responsible for building and repairing cell parts.
- Carbohydrates are responsible for providing quick energy.
- Enzymes are biological catalysts that speed up chemical reactions.
- Nucleic acids include DNA and RNA, which carry the cell’s genetic information.
Question 2
Which of the following lists the structures or processes involved in photosynthesis reactions in the correct order?
- Photosystem I > Photosystem II > Electron Transport Chain > Calvin Cycle
- Calvin Cycle > Photosystem I > Photosystem II > Electron Transport Chain
- Photosystem II > Photosystem I > Electron Transport Chain > Calvin Cycle
- Photosystem II > Photosystem I > Calvin Cycle > Electron Transport Chain
Correct Answer: 3
Explanations: The correct order is Photosystem II > Photosystem I > Electron Transport Chain > Calvin Cycle. Photosystem II was discovered and named after Photosystem I.
Question 3
Jasmine is out hiking and comes across a berry she does not recognize. She wants to know if it is safe to eat, so she pulls out her field guide and begins to identify the berry. Which taxonomic tool is Jasmine most likely using to do this?
- dichotomous key
- cladogram
- phylogenetic tree
- pedigree
Correct Answer: 1
Explanations:
- A dichotomous key features a series of two-choice (dichotomous) questions until the organism is narrowed down enough to identify.
- A cladogram would be too complicated to use because the differences listed are not always apparent.
- A phylogenetic tree shows evolutionary relationships, so it would not be practical to identify an unknown plant.
- A pedigree is used to show the inheritance of a trait, not to identify organisms.
Question 4
Which of the following choices lists the functions of muscle cells?
- hold tissues together, provide insulation and protection
- send and receive messages to and from the brain
- provide a barrier, aid in absorption, line internal surfaces
- move the skeleton, digest food, and pump blood
Correct Answer: 4
Explanations:
- Connective cells hold tissues together and provide insulation and protection.
- Nerve cells send and receive messages to and from the brain.
- Epithelial cells provide a barrier, aid in absorption, and line internal surfaces.
- Muscle cells attach to the skeleton to move it, move food through the digestive system to process it, and pump blood throughout the body.
Question 5
Which of the following is not an accurate descriptor of vascular plants?
- Vascular plants contain an internal transport system for water.
- Moss is an example of a vascular plant.
- Vascular plants can control water loss through their leaves.
- Vascular plants contain an internal transport system for food.
Correct Answer: 2
Explanations:
- Vascular plants have an internal transport system for water.
- Moss is a nonvascular plant.
- Vascular plants can control water loss through their leaves.
- Vascular plants contain an internal transport system for food.
Question 6
In which of the following phases of the cell cycle does the cell make a complete copy of its DNA?
- S phase
- G2 phase
- prophase
- cytokinesis
Correct Answer: 1
Explanations:
- In the S phase or synthesis phase, the cell creates a second complete copy of its DNA.
- In the G2 phase or second growth phase, the cell continues to grow and replicate organelles needed for cell division.
- During prophase, the chromosomes condense and a spindle forms.
- During cytokinesis, the cytoplasm splits and allows the cell to divide into two distinct cells.
Question 7
Which part of the brain is responsible for regulating homeostasis by connecting the nervous and endocrine systems and regulating body responses?
- thalamus
- amygdala
- hippocampus
- hypothalamus
Correct Answer: 4
Explanations:
- The thalamus serves as a relay station for the brain. It sorts through information that enters and leaves the cortex.
- The amygdala plays a role in governing emotional responses.
- The hippocampus is responsible for transferring information to long-term memory.
- The hypothalamus connects the nervous and endocrine systems. The nervous system receives and interprets signals, and the endocrine system provides the hormones and chemicals that bring about responses to those signals.
Question 8
Which of the following statements is true about the social systems of animals?
- Social systems may be complex and include many individuals, or they may be simple and include a single animal that lives independently.
- Social systems only include animals that live together in single-family units.
- Social systems refer to ranking systems between organisms that prey upon one another.
- Social systems are only for the emotional benefit of the animals involved.
Correct Answer: 1
Explanations:
- Lions and wolves have social systems that involve many individual members. Tigers have social systems in which they live independently and rarely interact with other tigers.
- Some social systems, like herds of cattle with many family units together in one large group, involve animals that do not live as a single family unit.
- Food chains are ranking systems between organisms that prey upon one another.
- Social systems provide protection from harm as well as emotional and social benefit.
Question 9
Which of the following terms refers to the process of pioneer plants breaking rock into soil, leading to the growth of small grasses, then small trees, large trees, and finally a variety of plant and animal species?
- secondary succession
- climax community
- primary succession
- tertiary succession
Correct Answer: 3
Explanations:
- Secondary succession takes place where there used to be plants previously.
- A climax community is the final result of ecological succession, featuring a variety of plant and animal species.
- Primary succession is typically a slow process and it takes place where no plants previously lived.
- Tertiary succession is not a type of ecological succession.
Question 10
What is the role of transfer RNA in translation?
- To carry amino acids and anticodons to the messenger RNA and build polypeptide chains.
- To link amino acids to one another in order.
- To provide a codon in a specific order to code for an amino acid.
- To signal the end of translation.
Correct Answer: 1
Explanations:
- Transfer RNA carries an anticodon with matching nucleotides for the codon. The corresponding amino acid at the other end of the transfer RNA is released when the anticodon is matched to the correct codon.
- Ribosomal RNA is responsible for linking the amino acids to one another.
- The messenger RNA provides the codons in a specific order.
- The stop codon signals the end of translation.
Subtest II: Chemistry Practice Test
Question 1
Which of the following is NOT a chemical reaction from real life?
- electricity production by windmills
- photosynthesis
- combustion in a diesel engine
- rusting of nails
Correct Answer: 1
Explanations:
- The conversion of wind energy into electricity is a physical process.
- Photosynthesis is a chemical process.
- Combustion is a chemical process.
- Rusting is a chemical process.
Question 2
Carbon tetrachloride, CCl
4
, is made during the following reaction: CS
2
+ 3Cl
2
→
CCl
4
+ S
2
Cl
2
.
How many moles of Cl
2
are needed to fully react with 4.2 mol CS
2?
- 2.8
- 8.4
- 1.4
- 12.6
Correct Answer: D
Explanations:
- The materials do not fully react with only 2 mol Cl2for each 3 mol CS
2
.
- The materials do not fully react with only 2 mol Cl2for each 1 mol CS
2
.
- The materials do not fully react with only 1 mol Cl2for each 3 mol CS
2
.
- From the reaction, it can be seen that 3 mol Cl2reacts with each 1 mol CS
2
. Set up a ratio to determine the needed number of moles of Cl
2
. 4.2 mol CS
2
✕
3 mol
Cl
2
1 mol
Cs
2
= 12.6 mol Cl
2
Question 3
Which chemicals are the reactants and which are the products in the following combustion reaction?
CH
4
+ 2O
2
→ CO
2
+ 2H
2
O
- Reactants: CH4and O
2
Products: CO
2
and H
2
O
- Reactants: CH4and CO
2
Products: O
2
and H
2
O
- Reactants: CO2and H
2
O
Products: CH
4
and O
2
- Reactants: O2and H
2
O
Products: CH
4
and CO
2
Correct Answer: 1
Explanations:
- The reactants, or chemicals present before the reaction, are on the left side of a chemical equation (CH4and O
2
) and the products, or chemicals present after the reaction, are on the right side of the equation.
- Reactants are on the left side of the arrow, products are on the right.
- This is reversed, CO2and H
2
O are the results of the reaction (products), the CH
4
and O
2
are the chemicals that react with each other (reactants).
- Reactants are on the left side of the arrow, products on the right.
Question 4
Which of the following represents a double replacement reaction?
- H2CO
3
→ H
2
O + CO
2
- NaOH + HCl → NaCl + H2O
- Zn + 2HCl → ZnCl2+ H
2
- Mg + O → MgO
Correct Answer: 2
Explanations:
- In this reaction, carbonic acid decomposes into water and carbon dioxide. This occurs when a carbonated beverage is opened and CO2bubbles out.
- This is a double replacement reaction. The hydroxide in sodium hydroxide (NaOH) is replaced with chlorine to form salt (NaCl), and the chlorine in hydrochloric acid (HCl) is replaced with the hydroxide to form water.
- This is a single replacement reaction. Zinc combines with 2 chlorine atoms from the hydrochloric acid (HCl) leaving zinc chloride (ZnCl2) and hydrogen gas (H
2
).
- This is a combination reaction in which magnesium is combined with oxygen to form magnesium oxide (MgO).
Question 5
Which of the following is NOT a concern related to the use of fission to generate electricity?
- explosions or leaks that release dangerous materials
- short lifetimes of fission power plants
- lack of available fuel
- storage of radioactive byproducts
Correct Answer: 3
Explanations:
- Explosions and leaks, such as that at Three Mile Island, are a concern.
- Fission power plants generally last only 20 years before they must be decommissioned.
- Lack of fuel is not currently a concern with fission powered electricity production.
- The half-lives of some fission byproducts are very long, so storage is a concern.
Question 6
Which of the following will increase the rate of dissolution of a solute in a solvent?
- increased temperature
- increased particle size
- an increase in solute mass
- increased density of the solvent
Correct Answer: 1, 2
Explanations:
- In most liquids, particles dissolve more quickly at higher temperatures.
- Smaller particles dissolve more quickly than larger particles.
- Increasing the mass of the solute does not speed its rate of dissolution.
- Increasing the solvent density does not increase the rate at which a solute dissolves.
Question 7
Which of the following elements has the lowest reactivity?
- Kr
- Fe
- Cu
- Ca
Correct Answer: 1
Explanations:
- Krypton (Kr) is a noble gas. Noble gases have the lowest reactivities of any elements. Reactivity is the ability of an element to chemically combine with another element and release energy. Other than the noble gases, elements on the outer edges of the periodic table are more reactive than elements in the center of the table.
- Iron (Fe) has a fairly low reactivity, but not as low as that of the noble gases.
- Copper (Cu) is more reactive than iron.
- Calcium (Ca) is near the left edge of the periodic table and is quite reactive.
Question 8
Which of the following correctly describes an endothermic reaction?
- surroundings are heated as the system releases heat and chemical bonds form
- surroundings are heated as the system releases heat and chemical bonds are broken
- surroundings are cooled as the system absorbs heat and chemical bonds form
- surroundings are cooled as the system absorbs heat and chemical bonds are broken
Correct Answer: 4
Explanations:
- This describes an exothermic reaction.
- A system must absorb heat to break chemical bonds.
- A system releases heat to the surroundings when chemical bonds form.
- An endothermic reaction absorbs heat from the surroundings as chemical bonds are broken.
Question 9
Which of the following is the currently accepted model of the atom?
- quantum model
- nuclear model
- planetary model
- plum pudding model
Correct Answer: 1
Explanations:
- Evidence today indicates that the quantum model is the most accurate.
- The nuclear model was an early theory proposed after Rutherford discovered the atomic nucleus.
- The Bohr model is often used today when drawing atoms; however, it is more simplistic than the atom indicated by today’s experiments.
- The plum pudding model was rejected after the discovery of the nucleus.
Question 10
Which of the following is emitted from the nucleus during beta decay?
- a helium nucleus
- one or more protons
- an electron
- pure energy; no protons, electrons, or neutrons
Correct Answer: 3
Explanations:
- Beta decay does not involve the emission of a helium nucleus.
- Beta decay does not involve the emission of protons.
- During beta decay, a neutron in the nucleus decays into a proton and an electron. The proton remains in the nucleus but the electron is emitted and carries energy away from the nucleus.
- Both particles and energy are emitted from the nucleus during beta decay.
Subtest II: Earth and Space Sciences Practice Test
Question 1
Which of the following observations first led to the big bang theory for the origin of the universe?
- low-temperature background radiation
- differing brightness of stars
- galactic redshifts
- pulsars
Correct Answer: 3
Explanations:
- Low-temperature background radiation was observed in the 1960s and further confirmed the big bang theory.
- Different star brightnesses is used today to determine the distance to some stars but did not lead to the big bang theory.
- In 1926 Edwin Hubble observed that the amount of redshift of galaxies varied with distance and concluded that the universe was expanding. This led to the development of the big bang theory.
- Pulsars are stars that become brighter and dimmer as they rotate. These are used today to determine the distance to some stars but did not lead to the big bang theory.
Question 2
What is the shape of the Milky Way Galaxy?
- spiral
- elliptical
- irregular
- ovoid
Correct Answer: 1
Explanations:
- The Milky Way is a spiral galaxy. Our solar system is located in one of the arms of the spiral.
- The Milky Way is not elliptical.
- The Milky Way is not an irregular galaxy; it has arms that spiral around the central core.
- Ovoid is not a name given to the shape of galaxies.
Question 3
How does hydrogen move through the Earth and the atmosphere?
- as part of the carbon cycle
- as part of the water cycle
- as part of the nitrogen cycle
- as part of the rock cycle
Correct Answer: 2
Explanations:
- Hydrogen is not a major part of the carbon cycle.
- Hydrogen, present in water, moves through the Earth and the atmosphere as part of the water cycle.
- Hydrogen is not a major part of the nitrogen cycle.
- Hydrogen is not a major part of the rock cycle.
Question 4
Which of the following rocks is sedimentary?
- bituminous coal, a soft, black, tarry rock formed from plant material
- gneiss, hard, coarse-grained rock with light and dark bands composed of different minerals
- pumice, very light rock with a frothy texture
- basalt, dark rock with small, uniform crystals
Correct Answer: 1
Explanations:
- Bituminous coal is formed by compaction of materials on Earth’s surface and is therefore sedimentary.
- Gneiss is metamorphic.
- Pumice is igneous.
- Basalt is igneous.
Question 5
Which of the following processes occurs when water in a river slows and drops sediments on the riverbanks?
- chemical weathering
- physical weathering
- erosion
- deposition
Correct Answer: 4
Explanations:
- Chemical weathering occurs when a chemical reaction breaks down a rock.
- Physical weathering occurs when a rock is broken down by physical means.
- Erosion occurs when rock particles are transported by wind or water.
- Deposition occurs when new sediments are laid down after having been eroded from another location. Sediments dropped by the river are deposits.
Question 6
Oxygen is stored in each of the following places. Which one is not typically considered part of the oxygen cycle?
- the mantle
- the atmosphere
- living organisms
- water
Correct Answer: 1
Explanations:
- Oxygen is stored in mantle rocks, but it is largely unavailable and is not considered a part of the oxygen cycle.
- The atmosphere is part of the oxygen cycle. Plants release oxygen to the atmosphere during photosynthesis.
- Living organisms are part of the oxygen cycle. Approximately 65% of the human body is oxygen. Organisms release oxygen when they decompose.
- Water, made of hydrogen and oxygen, is part of the oxygen cycle.
Question 7
Which of the following events best illustrates the principle of uniformitarianism?
- tectonic plate movement
- an earthquake
- a volcanic eruption
- global warming
Correct Answer: 1
Explanations:
- The principle of uniformitarianism states that the geologic processes occurring today are the same as they were in the past. By using this principle and measuring the current movement of tectonic plates, scientists can predict where the plates were in the past.
- An earthquake is a catastrophic event that changes the landscape in a short time.
- A volcanic eruption is a catastrophic event that changes the landscape and atmosphere in a short time.
- The current theory of climate change, formerly called global warming, appears to be caused by an excess of greenhouse gases in the atmosphere. This excess began with the Industrial Revolution and is not a process that occurred in the distant past.
Question 8
In which layer of the atmosphere is the ozone hole?
- troposphere
- stratosphere
- exosphere
- thermosphere
Correct Answer: 2
Explanations:
- The troposphere is the atmospheric layer in which we live and does not contain the ozone hole.
- Atmospheric ozone and the ozone hole are in located in the stratosphere.
- The exosphere is the outermost layer of the atmosphere and does not contain the ozone hole.
- The thermosphere is the layer of the atmosphere with the highest temperature but does not contain the ozone hole.
Question 9
What is the state of matter of the Sun’s core?
- plasma
- solid
- liquid
- gas
Correct Answer: 1
Explanations:
- The entire Sun, including the core, is a plasma. A plasma is a gas of nuclei and their electrons.
- No part of the Sun is solid.
- No part of the Sun is liquid.
- A gas consists of complete atoms (electrons bound to nuclei). The Sun is so hot that its atoms are completely ionized into plasma.
Question 10
Which of the following is an extremely bright galactic nucleus that may contain a black hole and is located in the center of a very old, very distant galaxy?
- neutron star
- red giant
- supernova
- quasar
Correct Answer: 4
Explanations:
- A neutron star is a very small, dense star left after a supernova when the star is not big enough to form a black hole. The core of a neutron star is tightly packed neutrons.
- A red giant is a phase of star life that occurs as the star begins to burn helium instead of hydrogen.
- A supernova is a violent explosion of a massive star.
- A quasar is a very old, very distant, bright galactic nucleus that likely has a black hole at its center.
Subtest II: Physics Practice Test
Question 1
A car traveling at 50 mph collides and locks bumpers with another car at rest. After the collision, the two cars move together in the forward direction. What type of collision occurred and was kinetic energy conserved during the collision?
- collision – inelastic, kinetic energy – not conserved
- collision – elastic, kinetic energy – not conserved
- collision – inelastic, kinetic energy – conserved
- collision – elastic, kinetic energy – conserved
Correct Answer: 1
Explanations:
- The cars locked bumpers so the collision was inelastic. Kinetic energy is not conserved during an inelastic collision.
- During an elastic collision, objects recoil without losing kinetic energy.
- Kinetic energy is not conserved during an inelastic collision.
- The collision was not elastic because the cars stuck together.
Question 2
How does a polarizing filter polarize light?
- The filter twists the oscillation direction of incident light rays.
- The filter absorbs all light rays not oscillating in a given direction.
- The filter transmits all light rays except those oscillating along its polarizing axis.
- The filter reduces glare from the surface of a lake.
Correct Answer: 2
Explanations:
- Polarizing filters do not twist the oscillation direction of the light. They only filter the rays that oscillate in the wrong directions.
- Unpolarized light oscillates in many directions. Polarized light oscillates in a single direction. A polarizing filter transmits only components that are parallel to the polarizing axis of the filter. The filter absorbs the rest.
- A polarizing filter only transmits light rays that oscillate parallel to its axis.
- Polarizing filters DO reduce glare, but that does not explain how they polarize light.
Question 3
Ms. Santiago teaches her students how to use a free-body diagram to determine the net force on an object when applying Newton’s second law. She shows the class a free-body diagram of a box that is subject to an upward force of F and a downward force mg. She asks the class to select, from the choices listed, the expression that represents the net force on the box:
Choice 1: F–mg
Choice 2: F+mg
Choice 3: ma
Choice 4: mg
The majority of the students select choice 3. Which of the following concepts should she reteach?
- In Newton’s second law, the expression for the net force is found from the vector sum of forces shown on the free body diagram.
- Force vectors that point in opposite directions are subtracted, not added when determining net force.
- The net force on an object is not equal to its weight.
- The weight of a box equals the product of its mass and the acceleration due to gravity.
Correct Answer: 1
Explanations:
A. Because they selected choice 3, Ms. Santiago realizes her students still think Newton’s second law (F = ma) is a simple equation to be solved by multiplying mass and acceleration. She should reteach the concept that the left side of this equation, the expression for the net force, is found by combining the forces that act on the object. These forces are shown in the free-body diagram.
B. The majority of her students did not select choice 2, so at this time they do not need another lesson on how to find a vector sum. This lesson may need to be reemphasized later.
C. The majority of her students did not select choice 4, the weight of the box, as the net force.
D. This lesson is about using a free-body diagram to find an expression for the net force, not about how to calculate an object’s weight.
Question 4
Momentum is a product of which of the following quantities?
- acceleration and time
- density and temperature
- mass and velocity
- force and distance
Correct Answer: 3
Explanations:
- The product of acceleration and time is not momentum.
- The product of density and temperature is not momentum.
- Momentum is the product of mass and velocity. Momentum is a vector quantity, meaning it has direction.
- The product of force and distance is work, not momentum.
Question 5
How do magnetic field lines show the difference between stronger and weaker magnetic fields?
- Lines are closer together when the field is strong.
- Lines are further apart when the field is strong.
- Lines are darker when the field is strong.
- Lines have more arrows on them when the field is strong.
Correct Answer: 1
Explanations:
- The number of field lines in a given area is directly proportional to the magnetic field lines. Lines close together indicate a strong field.
- Field lines that are farther apart indicate a weaker field.
- The darkness of the field lines does not correspond to the strength of the field.
- The number of arrows on the field lines does not correspond to the strength of the field.
Question 6
A thermometer is inserted into a glass of water and the temperature is measured. The process is repeated using the same thermometer in a box of sand. The two temperatures are equal. Which law of thermodynamics is represented in this scenario?
- the law of enthalpy
- the first law
- the second law
- the zeroth law
Correct Answer: 4
Explanations:
- The law of enthalpy is not one of the three laws of thermodynamics.
- The first law equates a change in internal energy with the amount of heat added and work done.
- The second law states that entropy increases in any process.
- The zeroth law states that if 2 objects are in thermal equilibrium with a 3rd object (the thermometer), then they are in equilibrium with each other.
Question 7
In order for an object to emit electromagnetic radiation, its temperature must be:
- at least as high as the temperature of a star.
- above absolute zero.
- at least as high as the temperature of a campfire.
- at least as high as the human body temperature.
Correct Answer: 2
Explanations:
- Stars are hot enough to emit visible light, x-rays, and gamma rays, but cooler objects can emit lower energy electromagnetic radiation.
- Objects at all temperatures above absolute zero emit electromagnetic radiation.
- Campfires emit visible light but cooler objects can emit lower energy electromagnetic radiation.
- The human body emits infrared light but cooler objects can emit lower energy electromagnetic radiation.
Question 8
After having her students wash their hands, Ms. Akito distributes cups of small dried fruits and a clean paper towel to each student. She asks her students to count and record the initial number of fruits, and return the fruits to the cup. Every three minutes she asks her students to pour the fruits onto their paper towel and eat or dispose of half of the fruits, record the number remaining, and return the remaining fruits to their cups. After all the fruits are gone, the students graph the number of remaining fruits versus time. Which concept is Ms. Akito most likely teaching through this activity?
- half-life
- isotope formation
- alpha decay
- age of the earth
Answer: 1
Explanations:
- During one half-life, half of the atoms in a sample decay, on average. Ms. Akito is teaching the concept of half-life during radioactive decay by having students dispose of half of the fruits at the end of each time interval.
- Isotopes are atoms with identical numbers of protons but different numbers of neutrons. Ms. Akito is not teaching about isotopes.
- Ms. Akito is not teaching about alpha decay.
- Ms. Akito is most likely not teaching about the age of the earth.
Question 9
A wave with an amplitude of 7.5 m interferes with a wave of amplitude 2.5 m. What is the amplitude during a moment of destructive interference?
- 2.5 m
- 10 m
- 7.5 m
- 5 m
Correct Answer: 4
Explanations:
- The amplitude of destructive interference does not equal the smaller amplitude.
- The amplitude during constructive interference is 10 m.
- The amplitude of destructive interference does not equal the larger amplitude.
- The wave amplitude during a moment of destructive interference is the difference between the two individual wave amplitudes or 5 m.
Question 10
Select from the following list all of the problems with the planetary model of the atom.
- It did not explain why electrons do not fall into the nucleus.
- It did not model the behavior of heavier elements.
- It proposed that atoms are the smallest particles of matter.
- It did not allow for the presence of neutrons in the atom.
Correct Answers: 1, 2
Explanations:
- The planetary, or Bohr, model of the atom gives no explanation of why electrons remain in orbit around the nucleus.
- The planetary model does not correctly explain heavier elements.
- The planetary model does allow for particles smaller than atoms.
- The planetary model does not prohibit the existence of neutrons in the nucleus of the atom.