Praxis️ Middle School Science Category III: Physical Sciences
Overview
This content category has 28 selected-response questions. These questions account for 22% of the entire exam. Check out the competencies:



Let’s talk about some key concepts that are likely to be addressed in this section.
Work
Work is what causes things to move or change position. Work is calculated by the equation W = F ∙ d cos????. F is the force applied, d is the displacement, and ???? is the angle between the force and the displacement. Work only occurs in the direction of the movement. Note that if the force vector and the displacement vector are perpendicular to each other, no work happens because cos(90) is 0. Any quantity multiplied by 0 is also 0. The unit of measurement for work is a joule, also known as a newton meter.
Some sample problems are as follows:
How much work is used to push a box up a 5-meter-long ramp angled at 60 degrees with the horizon if a student uses a 15 N force applied horizontally?
W = F ∙ d cos????
= 15 ∙ 5cos(60)
= 15 ∙ (5 ∙ ½)
= 45 joules
How much work is necessary to lift a 10 kg box 10 meters? Assume the force is applied in the direction of movement.
The force which must be applied is 10kg ∙ 10 m/s2 = 100 N.
The distance traveled is 10 meters.
The angle is zero.
W = F ∙ d cos????
W = 100N ∙ 10 meters ∙ cos(0)
W = 100N ∙ 10 meters ∙ 1
W = 1000 joules
Types of Waves and Basic Characteristics
There are several ways to categorize waves.
- Categorize by the direction of the motion:
- Longitudinal waves are waves in which the motion of the particles is in the same direction as the motion of the energy. A sound wave is an example of a longitudinal wave.
- Transverse waves are waves whose motion is perpendicular to the direction of energy movement. For example, a Slinky spring toy moving up and down would represent a transverse wave.
- Surface waves are waves in which the motion of the particles is circular.
- Categorize based on whether they can transmit energy through a vacuum:
- Mechanical waves cannot transmit energy through a vacuum. Examples of this principle include sound waves and water waves.
- Electromagnetic waves can transmit energy through a vacuum. There are several different types of electromagnetic waves, as illustrated below.
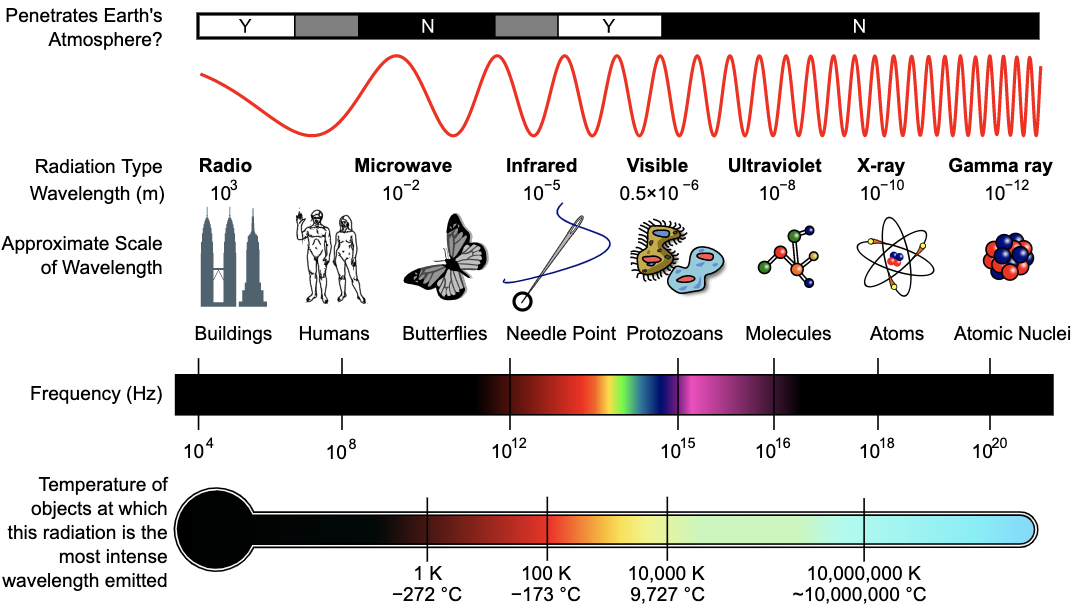
Inductiveload, NASA [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/)]
All waves have common features. Frequency refers to how fast the wave completes a cycle. Amplitude refers to the strength or height of the wave. Wavelength (ƛ) is the distance between corresponding parts of the wave. Speed is how quickly the wave travels. Intensity is the power per area and is used to measure mechanical waves.
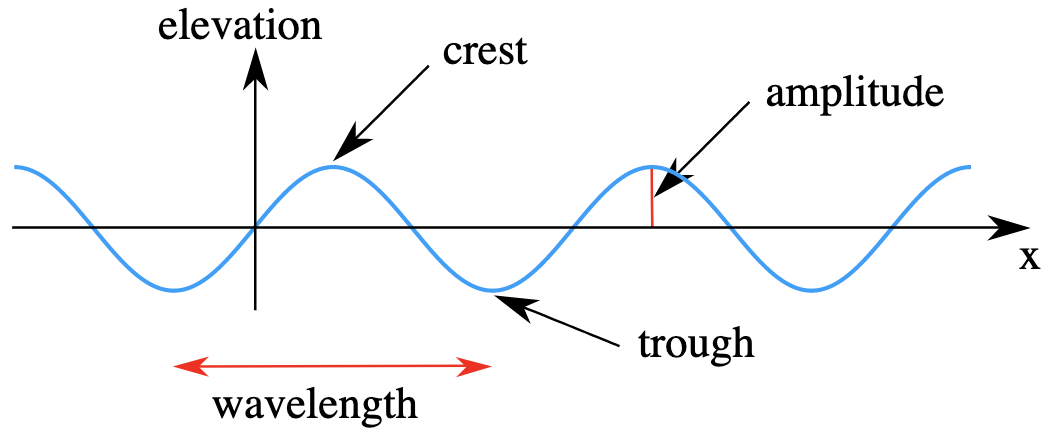
Kraaiennest[CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
The frequency of the wave is measured in hertz and is calculated by c/ƛ.
How to Balance Chemical Equations
There are four basic types of chemical reactions.
- Synthesis – when two or more elements or compounds combine into a more complicated compound
- A + B → AB
- Decomposition – when a compound breaks into smaller components
- AB → A + B
- Single replacement – when one element replaces another in a compound
- AB + C → AC + B
- Double replacement – when two elements swap places in two compounds
- AB + CD → AD + CB
All chemical reactions are balanced in that they observe the law of conservation of mass.
How to balance the notation of a chemical reaction:
- Count the number of atoms of each element on both sides of the chemical equation
- Rules for counting atoms
- Coefficients apply to all elements following the coefficient. Example: 2HF has 2 hydrogens and 2 fluorines
- Subscripts apply only to the element immediately preceding them. Examples: H2O has 2 hydrogens and one oxygen. CCl4 has 1 carbon and 4 chlorines.
- Parentheses are used to group polyatomic ions. Polyatomic ions can be balanced as a unit because they are grouped in nature. Pb(NO3)2 has one lead and two nitrate groups.
- Determine if anything is imbalanced. If so, make changes to balance atoms.
- Change coefficients in front of compounds to change atom numbers.
- Re-count atoms of each element until there are the same number of atoms of each element on both sides of the equation.
Example 1
Balance the following equation:
N2 + H2 → NH3
The reactants side has 2 nitrogens and 2 hydrogens. The products side has 1 nitrogen and 3 hydrogens. To balance hydrogen, the least common multiple of 2 and 3 is 6. So place a 3 in front of the reactants hydrogen, and a 2 in front of the products:
N2 + 3H2 → 2NH3
Re-count both sides: everything is balanced! There are 2 nitrogens on each side and 6 hydrogens on each side.
Example 2
Balance the following equation:
Pb(NO3)2 + NaCl → NaNO3 + PbCl2
The left side has 1 lead, 2 nitrates, 1 sodium, and 1 chlorine. The right side has 1 sodium, 1 nitrate, 1 lead, and 2 chlorines.
Add a 2 in front of NaCl on the left-hand side to balance chlorine:
Pb(NO3)2 + 2NaCl → NaNO3 + PbCl2
Re-count: The left side has 1 lead, 2 nitrates, 2 sodiums, and 2 chlorines. The right side has 1 sodium, 1 nitrate, 1 lead, and 2 chlorines.
Add a 2 in front of NaNO3 on the right to balance sodium:
Pb(NO3)2 + 2NaCl → 2NaNO3 + PbCl2
Re-count: The left side has 1 lead, 2 nitrates, 2 sodiums, and 2 chlorines. The right side has 2 sodiums, 2 nitrates, 1 lead, and 2 chlorines. They’re balanced! You’re done!
Gas Laws
There are several laws that describe the behavior of gases.
Boyle’s law states that when the temperature is held constant, pressure is inversely proportional to volume. That means that if you increase the pressure on a gas at a given temperature, the volume will decrease to compensate: P1 x V1 = P2 X V2. In other words, the product of pressure times volume remains constant.
Charles’s law states that the volume and temperature are directly proportional: V1 / T1 = V2 / T2. For example, at constant pressure, a balloon that is adequately inflated with air at room temperature will deflate if placed in a freezer for an extended period of time.
Amonton’s Law (aka Gay-Lussac’s law and Dalton’s law) states that when the volume is held constant, the pressure is proportional to temperature.
Solution Terminology
There are many terms used to describe solutions. A solution is a mixture in which a solute is dissolved in a solvent. A common everyday solution is saltwater. In saltwater, the solute is the NaCl, while the solvent is water.
There are informal terms related to solutions. A “dilute” solution is weak and doesn’t have much dissolved solid. (The term “dilute” can also be used as a verb, meaning to add more solvent.) A “concentrated” solution is strong and has a large amount of dissolved solute.
The terms saturated, unsaturated, and supersaturated relate to how much solute is dissolved in a given amount of solid. A solution is saturated if it has the maximum amount of solute possible while still remaining stable, an amount that is visually represented on the solubility curve. It is supersaturated if it has more than the maximum stable amount of solute. Supersaturated solutions are created by making the solution at a high temperature and slowly cooling them. A solution is unsaturated if it is below the solubility curve. This means more solute can be added to the solution.
Molarity is the number of moles of solute per liter of solution.
Molality is the number of moles of solute per kilogram of solvent.
And that is some information about the Physical Sciences category of the Praxis️ Middle School Science exam.
